NASA made some pretty neat posters. Share and enjoy.
So let's say, you've got some compelling reason to mine uranium on Mars. Perhaps a certain deranged Urth president has imposed crippling tariffs on your precious fission fuels, and your atomic rockets' fuel rods are steadily becoming depleted. You can scoop up propellant with a shovel and put it into the propellant tanks, but without the atomic pile to work its hot magic you're going nowhere. What do you do? Well, after rolling into a ball and crying a lot, you can dig out the geology maps for Mars because fortunately it's one of the few places in the solar system besides Earth that you can find anything more than the poorest-grade ores. People tend to assume that asteroids are full of everything from platinum to antimatter and rainbows - they're not.
In theory you can process almost any amount of a given rock and get something out of it - but processing a million tonnes of rock to get even one tonne of uranium is ridiculously uneconomical. Therefore, as with most minerals, you go looking for where it's concentrated.
There are two sorts of questions one has to ask about the possibility of uranium sources besides the Earth. The first question is, is the planet or asteroid core differentiated? That is, was it molten long enough for the elements to separate out into a heavy core, mantle and crust floating on top? The second is, was there some way for the uranium to be concentrated?
Before we talk about that, let's talk about what uranium is like - it's a "lithophile" element, like the rare earths and also thorium. Rare earths go by certain rules, which also sort of apply to U and Th. It makes them a useful geochemical marker, and you can often find uranium where you find rare earths, but that's a loose relationship. So when we talk about finding rare earths we're also talking about the fissile fuels, thorium and uranium. Rare earth tailings are often slightly radioactive because of the leftover U and Th.
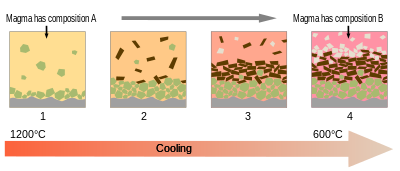
But why is it so difficult to get a hold of the rare earths? Well, for a start you need some kind of separation process, and rare earths are notoriously difficult to concentrate in "veins" and so on. A magma sea allows molten minerals to precipitate out of solution and become concentrated. Generally, as magma cools other minerals and elements leave the magma party first by dissolving out as crystals. The rare earth elements are the hard partiers who you can't really get to leave. Eventually, by repeated heating and cooling, all that's left are the rare earths and you get some high purity stuff, along with tough crystals like garnet. But usually that's not the case and you get a mishmash of different minerals with some enrichment of rare earth elements. Generally, you know when a mineral is not going to hang out with rare earths because they are just incompatible due to electron valences etc. For example, olivine is always going to have very little in the way of rare earths.
I'm sure there's a Steven Universe joke in here somewhere.
Most asteroids are never heated to this degree or if they are, are not molten long enough for this to happen. So, they are a random collection of accreted space dirt. You won't find any ore concentration processes happening on them. No veins of uranium unless there happens to have been hit by a freak uranium-rich rock, which will probably turn out to be a derelict atomic rocket. Typically, the concentrations of U in asteroids are in the ranges of less than 1 part per million, which is pathetic as far as ores go. Marginally economic uranium ores on Earth are 100 times richer. This is also why you can't economically mine lunar rare earths - at best they are equivalent to low-grade ores on Earth. So forget it. Nothing short of desperation or some clever way to recover it as byproduct is going to make it worthwhile over simply ordering it from alibaba.com.
Now, onto the next stage of concentration, which typically involves the use of liquid water - not something you'll ever find on an asteroid.
In general, uranium is leached out of rocks when water is oxidative, that is, has an excess of electrons to give away. Compounds like H202 and the notorious perchlorates widely present on Mars can do this. The dissolved uranium then precipitates out under acidic conditions, as would be the case in the drying Martian seas. Then, as it is eroded to sand, it can be sorted by wind and wave action, for example. Processes like this are what makes uranium and rare earths start to part ways, especially the lighter rare earths like lanthanum. There are a large number of ways uranium can become concentrated. It can even be concentrated in plant matter, becoming coal.
Mars had both a differentiated core and the possibility of hydrothermal action. Heck, we know Mars was wet thanks to the various Mars rovers. And, as the planet became dryer, the water became more and more acidic: the right conditions for uranium to precipitate out into deposits.
Wind action, presumably when the atmosphere was more dense, would have worked with wave action to sift sands and gradually shuffle the heaviest stuff to the bottom. This is how mineral sands work. As Mars' seas gradually shrank, the stuff would wind up left behind on the dry seabed.
Martian thorium concentrations. The red zone is about 1ppm.
Thorium is often found in association with uranium, and a useful nuclear fuel in its own right. It has quite a strong gamma decay signal and so is fairly easy to pick up a gamma spectrometer in orbit. The Mars Odyssey orbiter picked up quite a strong concentration of the stuff in Chryse Planitia just where a shrinking ocean would have receded to.
These are probably monazite sands, which are eroded out of granitic rocks which have been subject to fractional crystallisation (that process mentioned above). This was probably fed by volcanic eruptions and lavas from Pavonis Mons etc. to the south-west.
Along with monazite grains, fluorine-enriched minerals from Mars have been found, and even fluorapatite. This is nice because we can make uranium hexafluoride with the fluorine, an important part of the enrichment process (you can't just shovel uranium-rich sand into a nuclear reactor after all). Fluorine also makes a terrifyingly effective rocket fuel. And by terrifying, I mean the rocket engineers have not been able to make a fluorine-based rocket engine that didn't blow up. Fluorine is also necessary for a number of plastics, as well as teflon, used in liquid hydrogen tankage and plumbing and of course non-stick frying pans.
Fluorapatite - Ca5(PO4)3F. Like most minerals, usually occurs just as tiny crystals mixed in with other stuff.
Other potential sources include iron-oxide rich laterite clays, as well as certain carbonate rocks. Laterite clays are normally only found in tropical conditions on Earth, but are expected to be common on Mars. The nice thing about have sands as opposed to veins is that the sands are easy to mine. Just scoop them up - no rock crushing required, which requires extremely heavy duty machinery. Clays are also quite soft and require minimal processing. The rare earths, thorium and uranium can be chemically leached out of the rock with a series of hot acidic and alkaline solutions. It won't be the most efficient operation, but then again you are not trying to sell the stuff on the Earth market - possibly because the Urth-people have already nuked themselves into oblivion.
Perhaps the Asteroid Belters will come and trade rare heavy metals like platinum and rhenium for uranium and dysprosium (an important rare earth for high-strength electric motors).
Perhaps the Asteroid Belters will come and trade rare heavy metals like platinum and rhenium for uranium and dysprosium (an important rare earth for high-strength electric motors).
This handy overview of Martian habitability for both human explorers and potential Martian life shows that the best locations with ice are mostly above or below 30 degrees latitude, which is also where solar power becomes less useful and temperatures drop. Fortunately the Th concentrated regions shown above in Chryse Planitia will have some subsurface ice to support the miners without being too far north as to expose them or their machinery to freezing long Martian winter nights.
So, there you have it. As far as uranium is concerned, there's no place like home (Earth) - but you can at least ask the Martians if they have some.



No comments:
Post a Comment